Valeritas’ V-Go® Wearable Insulin Delivery Device Demonstrates a Reduction in A1c Levels by 1.5 and Total Daily Insulin Dosage by 14%
Share:
BRIDGEWATER, N.J., April 26, 2019 (GLOBE NEWSWIRE) -- Valeritas Holdings, Inc. (NASDAQ: VLRX), a medical technology company and maker of V-Go® Wearable Insulin Delivery device, today announced results from the VERDICT study at the American Association of Clinical Endocrinologists (AACE) Annual Meeting. The VERDICT study demonstrated that patients with type 2 diabetes who switched to V-Go can improve their average blood sugar levels (A1c) with less insulin.
VERDICT is a real-world retrospective analysis conducted by Dr. Trisha Zeidan, Premier Physician Network, Bull Family Diabetes Center. The study utilized electronic health records from a large specialized diabetes center to identify patients exceeding glycemic targets (A1c > 7.0) prescribed insulin therapy or naïve to insulin at baseline, and who changed to V-Go between January 2013 and December 2016.
“The efficacy and insulin usage results from the VERDICT study demonstrate both the medical and economic value of V-Go for patients with type 2 diabetes prescribed insulin,” said John Timberlake, President and Chief Executive Officer of Valeritas. “Diabetes has become a major healthcare issue and we have demonstrated through strong, real-world evidence the use of V-Go by patients with type 2 diabetes can reduce not only their A1c levels but also lower their total daily insulin usage.”
This retrospective analysis of 139 patients with type 2 diabetes evaluated the effect V-Go had on patients’ A1c, insulin total daily dose (TDD), concomitant non-insulin diabetes medication, and reported hypoglycemia. Patients using V-Go had an average reduction in A1c levels of -1.5 (p<0.01) with a decrease in insulin TDD of 14% (p<0.01). In addition, the percent of patients prescribed concomitant medications decreased and documented hypoglycemia decreased from baseline.
About Valeritas Holdings, Inc.
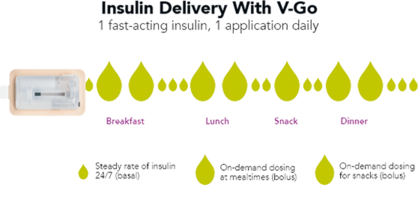
Valeritas is a commercial-stage medical technology company focused on improving health and simplifying life for people with diabetes by developing and commercializing innovative technologies. Valeritas’ flagship product, V-Go® Wearable Insulin Delivery device, is a simple, affordable, all-in-one basal-bolus insulin delivery option for patients with type 2 diabetes that is worn like a patch and can eliminate the need for taking multiple daily shots. V-Go administers a continuous preset basal rate of insulin over 24 hours, and it provides discreet on-demand bolus dosing at mealtimes. It is the only basal-bolus insulin delivery device on the market today specifically designed keeping in mind the needs of type 2 diabetes patients. Headquartered in Bridgewater, New Jersey, Valeritas operates its R&D functions in Marlborough, Massachusetts.
More information is available at www.valeritas.com and our Twitter feed @Valeritas_US, www.twitter.com/Valeritas_US.
Investor Contacts:
Lynn Pieper Lewis or Greg Chodaczek
Gilmartin Group
646-924-1769
ir@valeritas.com
Media Contact:
Kevin Knight
Knight Marketing Communications, Ltd.
206-451-4823
pr@valeritas.com




