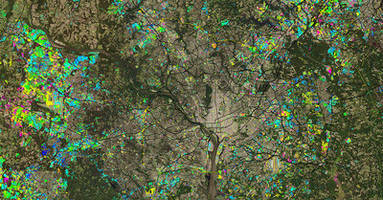
Maxar Selected to Provide its NaturalVue-® 2.0 Image Mosaic and Urban Change Indicator Products to Esri
WESTMINSTER, CO, March 28, 2019 /CNW/ - Maxar Technologies (NYSE:MAXR) (TSX:MAXR), a global technology innovator powering the new space economy, today announced an agreement to integrate its NaturalVue® 2.0 image mosaic and National Urban Change Indicator (NUCI) commercial products into ArcGIS Living Atlas of the World. The operations of DigitalGlobe, SSL and Radiant Solutions were unified...
Read More »
New eBOX671-521-FL Embedded Vision System Supports MXM 3.1 type A Graphics Module
Applications are machine vision, edge computing, traffic vision, deep learning, and artificial intelligence of things. Comes with I/O interfaces, two RS-232/422/485, one DVI-I port, one HDMI port, one VGA port, and two DisplayPort outputs. Features 8th gen Intel® Core™ i7/i5/i3 & Celeron® with Intel® Q370 chipset (Coffee Lake-S).
Read More »Acculogic Will Show Advanced Test Equipment at the Space Tech Expo
Markham, ON - Acculogic Inc., a global leader in electronic production test solutions, announces that it will exhibit in Booth #4031 at the Space Tech Expo, scheduled to take place May 24-26, 2016 at the Pasadena Convention Center in Pasedena, California. The company’s Test Engineering division offers a comprehensive range of turn-key services that range from in-circuit test fixture and program...
Read More »High Definition Telescope offers narrow band imaging.
Utilizing Extra-Low Dispersion Glass for rigid surgical endoscopy, FDA-cleared OES Elite 4mm HD Telescope can be used for urological procedures in resection, including transurethral resection of bladder tumor procedures, which are used both to visualize bladder cancer and to remove cancerous tissue. Narrow Band Imaging works by filtering white light into specific light wavelengths that are...
Read More »
Common Misconceptions About Custom Automation Systems
This guide will help you understand the capabilities of automation systems.
Read More »Visage Leads with Enterprise Imaging at ACR 2016
Visage's market leadership continues with the recent signing of two large health systems SAN DIEGO, Calif.,Ã- – Visage Imaging Inc. ( Visage ), a wholly owned subsidiary of Pro Medicus Ltd. (ASX: PME), has announced they will be exhibiting at the American College of Radiology 2016 Annual Meeting (ACR 2016, Washington, DC, May 16-17, Booth #105/204), demonstrating the latest release of the...
Read More »
WatchGuard Video Answers Demand to Strengthen Police Video Evidence
VISTA WiFi Developed for Law Enforcement Agencies Seeking Integration of In-Car and Body-Worn Video Camera Systems ALLEN, TexasÃ- – Video evidence and the ability to aggregate it has become an integral part of effective law enforcement and the justice system. In a recent interview, Allen Cummings, Chief of Police in Pennsville, New Jersey, a small community on the banks of the Delaware...
Read More »Mopec Signs Exclusive Imaging Agreement with Milestone
'Industry’s first touch screen grossing station will feature Milestone gross imaging camera and software’ OAK PARK, Mich. - Mopec, creator of the industry’s first touch screen grossing station, announced today it has signed an exclusive agreement to feature Milestone’s MacroPATH pro-x macro digital imaging system with its MB800 grossing station. Milestone’s pro-x camera system can now...
Read More »
Mahr Federal to Feature MarVision MM 320 and MarVision QM 300 Video Measuring Microscopes with Image Processing at MD&M EAST 2016
Also on display will be the MarShaft™ SCOPE 250 plus flexible optical system for shop floor measurement PROVIDENCE, RI – Mahr Federal will feature the MarVision MM 320 and the MarVision QM 300 video measuring microscopes with image processing capability at MDM EAST, June 14-16, 2016, Jacob K. Javits Convention Center, New York, NY, Booth #2121. Designed for the measurement and/or dimensioning...
Read More »
Prone Biopsy System provides 360-degree breast access.
Offering both 2D and 3D™ imaging-guided breast biopsies, Affirm™ allows radiologists to better target lesions found during 3D MAMMOGRAPHY™ exams, as well as other screening modalities. With patient lying prone, system provides true 360Ã-
Read More »
Ruggedized Mobile Terminals feature smartphone-based design.
With MX-1000 Series, organizations can perform tasks such as inventory management, logistics, and field service. Mobile terminals combine flexibility of off-the-shelf smartphones with rugged hand-held assembly that holds both phone and barcode reader. Vision-enabled units can read 1-D and 2-D label-based codes as well as challenging 2-D DPM codes. Accepting variety of AndroidÃ-® and...
Read More »
Do You Have Unique Lifting Needs?
EZRig Cranes offers lifting solutions that are engineered for robust performance, providing the ultimate in portability. Designed for real-world lifting needs, our products are small and light with options and features that make them ideal for a broad range of applications. See our video to learn how an EZRig Crane can simplify your lifting requirements.
Read More »



