As of 2017, CO2 molecules only accounted for about 405 out of every million molecules of air — that is, 405 parts per million (ppm). Why do climate scientists place so much importance on the role of carbon dioxide (CO2) in human-caused global warming? Isn’t CO2 just a trace gas? How could a gas with such small concentration cause the earth’s temperatures to rise, resulting in environmental destruction?
A report from the National Academy of Sciences asserts, “Emissions of carbon dioxide from the burning of fossil fuels have ushered in a new epoch during which human activities will largely determine the evolution of the Earth’s climate. Because carbon dioxide is long-lived in the atmosphere, increases in this key gas can effectively lock the Earth and many future generations into a range of impacts, some of which could be severe.
"Therefore, emission reduction choices made today matter in determining impacts that will be experienced not just over the next few decades, but also into the coming centuries and millennia,” the report notes.
Exploring the CO2 Molecule
What is it about the CO2 molecule that causes such a fuss?
Climate scientists focus on carbon dioxide because it is a greenhouse gas and contributes to the greenhouse effect. The use of the word “greenhouse” in this context sometimes confuses people. Here’s what it means: The transparent glass in a greenhouse roof lets solar radiation into the building. In a similar way, the earth’s transparent atmosphere lets solar radiation make it down to the Earth’s surface.
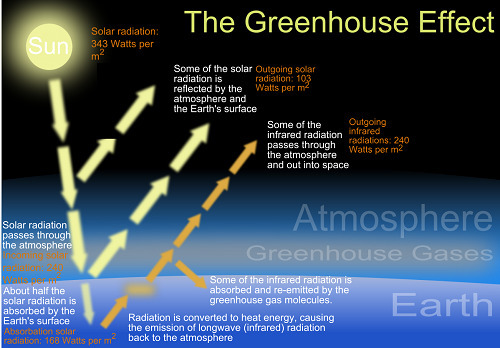
Both systems — the glass greenhouse and the earth’s greenhouse effect — trap heat and keep the occupied space warm. But they do so for different reasons. The glass greenhouse traps warm air that has been heated by the solar radiation; without the glass, the warm air would rise and blow away. But the Earth’s atmosphere doesn’t just hold warm air; it actually absorbs the radiation. Molecules in the atmosphere take in the energy being radiated up from Earth’s surface.
In reality, Earth’s greenhouse effect is a good thing. If not for the greenhouse effect, Earth’s average surface temperature would be -18 °C or -40 °F. Instead, our planet is a livable 15 °C, or 59 °F. Climate scientists have concluded, though, that human activity is increasing the greenhouse effect and that this could have negative effects on overall living conditions.
So, how does it work?
How Does CO2 Trap Radiation?
Put simply, carbon dioxide likes a certain kind of radiation and is very good at capturing it.
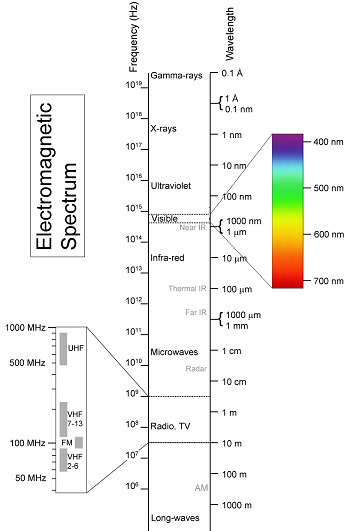
The sun emits electromagnetic radiation of all wavelengths. About 50% of the sun’s radiation is visible light; the spectrum of visible light goes from .4 microns to .7 microns (abbreviated as µm, a micron being a micrometer or one-millionth of a meter).
Radiation, including visible light, travels in waves; wavelength is the distance from the crest of one wave to the crest of the next. The visible light spectrum, which you can observe in a rainbow or a prism, goes from violet at .4 µm through blue, green, yellow, orange, to red at .7 µm. To clarify, 0.4 µm is a shorter wavelength, and .7 µm is a longer wavelength. About 10% of the sun’s radiation, that radiation below the visible spectrum, is shorter-wavelength, or ultraviolet principally. The rest, about 40%, is above the visible spectrum, mostly longer-wavelength infrared radiation.
So the sun is constantly blasting Earth with this radiation across the spectrum, especially visible and infrared. The energy that makes it to the earth’s surface gets absorbed and then radiated back out. The greenhouse effect works in part because Earth is much cooler than the sun, so when Earth radiates, it tends to send out lower-energy, longer-wavelength infrared radiation.
Why Does CO2 Absorb Infrared Radiation?
Greenhouse gases, especially CO2 and water vapor, are very good at absorbing infrared radiation, particularly around 15 µm. Eventually, almost all of the radiation in the Earth’s atmosphere gets reradiated back out into space, but the greenhouse gases hold it in long enough to generate a greenhouse effect in the planet’s atmosphere and keep us at a comfortable 15 °C.
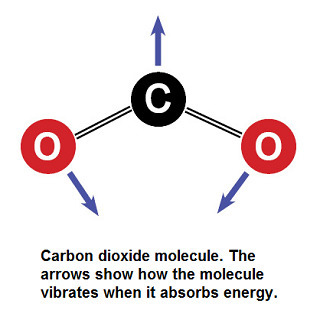
What’s going on in a CO2 molecule when it absorbs energy?
David Archer, professor of Geophysical Sciences at the University of Chicago, writes, “Gases are the simplest type of molecule, and they only vibrate in very particular ways. Vibrations in a gas molecule are like vibrations of a piano string in that they are fussy about frequency. This is because, like a piano string, a gas molecule will only vibrate at its ‘ringing’ frequency.
When you get down to the molecular level, it becomes useful to think of radiation as traveling in pulses, or ‘photons.’ When a photon hits a molecule, it can cause it to vibrate, to take on the photon’s energy, absorbing it. CO2 vibrates by bending in the direction of the arrows shown in the following illustration. Not just any old photon can get absorbed by a CO2 molecule — it has to be a photon of radiation within certain wavelengths. Any photon that is not within those relatively narrow wavelengths will just pass by the CO2 molecule and go look for some more congenial greenhouse gas to take up with, or maybe just mosey on up into space.”
The ESPERE (Environmental Science Published for Everybody Round the Earth) website provides a helpful analogy that helps explain how energy absorption by greenhouse gases works.
“Imagine that you are playing miniature golf,” it says, "and you come to one of those maddening holes where the cup is right at the peak of a hill. If you putt the ball too softly, it will roll back down the hill and everyone will laugh at you. If you putt the ball too hard, it will skip over the hole, resulting in more hilarity. But if you putt the ball with just the right force, it will drop neatly into the cup at the top of the hill, and everyone will admire you.”
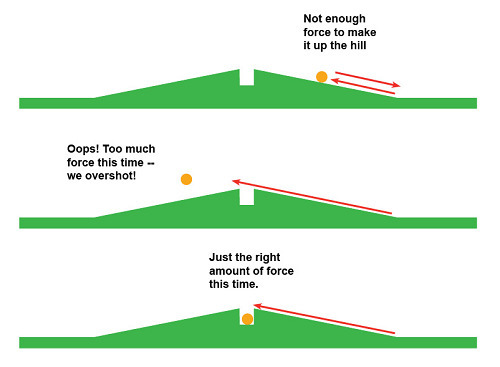
In a similar way, only photons with certain energy characteristics can be absorbed by a CO2 molecule (or any other greenhouse gas).
The greenhouse gases receive a constant bombardment of infrared radiation emitted from the earth. Their molecules vibrate, heating up the air.
The Greenhouse Gases
Carbon dioxide is only one of the greenhouse gases. Water vapor, methane, nitrous oxide, ozone, and freon-11 and -12 are greenhouse gases, as well. However, CO2 stays in the atmosphere longer than the others. For example, water vapor is the most abundant greenhouse gas, but it condenses and only stays in the atmosphere for a few days, so it doesn’t build up in the atmosphere as CO2 does.
The Union of Concerned Scientists (UCS) says, “In the case of CO2, much of today’s emissions will be gone in a century, but about 20 percent will still exist in the atmosphere approximately 800 years from now. This literally means that the heat-trapping emissions we release today from our cars and power plants are setting the climate our children and grandchildren will inherit.”
The following chart from the UCS shows that, of all human causes, CO2 emissions exert the most influence on global warming.
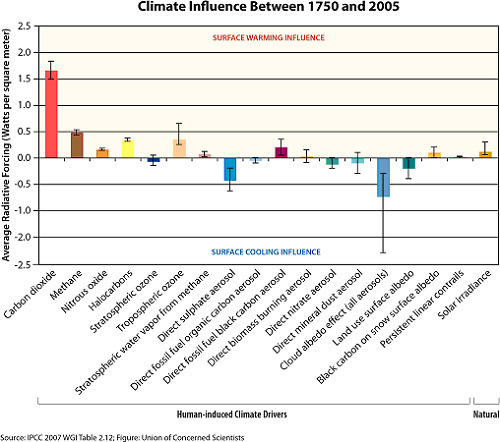
Climate scientists are concerned that CO2 concentrations in the atmosphere have risen from 280 ppm in pre-industrial times to 405.0 ppm as of 2017. That increased concentration has caused an imbalance in the greenhouse effect, so that the atmosphere is capturing a little extra energy each year, raising global surface temperatures.
Research by NASA’s Goddard Institute for Space Studies (GISS) has identified CO2 as “The Thermostat that Controls Earth’s Temperature.” A science brief by NASA scientist Andrew Lacis emphasizes that, while both water and CO2 are greenhouse gases, they provide different functions in the maintenance of the greenhouse effect. Water vapor condenses, whereas CO2, methane, ozone, and the other trace gases are noncondensing.
Lacis writes, “Water vapor accounts for about 50% of the Earth’s greenhouse effect, with clouds contributing 25%, carbon dioxide 20%, and the minor greenhouse gases (GHGs) and aerosols accounting for the remaining 5%. … Thus, while the non-condensing greenhouse gases account for only 25% of the total greenhouse effect, it is these non-condensing GHGs that actually control the strength of the terrestrial greenhouse effect since the water vapor and cloud feedback contributions are not self-sustaining and as such, only provide amplification. Because carbon dioxide accounts for 80% of the non-condensing GHG forcing in the current climate atmosphere, atmospheric carbon dioxide, therefore, qualifies as the principal control knob that governs the temperature of Earth.”
The Takeaway
So, how can a trace gas that makes up only 405.0 ppm of the earth’s atmosphere possibly make any difference? How can the CO2 molecule have such a great effect on the planet?
The upshot is that even a relatively small concentration of a substance can have a big impact — especially at large scales and over long periods.
This article was originally written by Al Bredenberg in 2012 and was updated by Helen Carey in 2019.

