VisiPak, a Sinclair & Rush, Inc. Company, Introduces New Face Shield Designs: Different Comfort Features and Coverage Levels are Now Available
Press Release Summary:
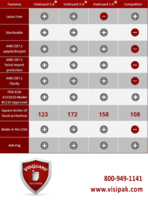
- All VisiGuard® designs are ANSI Z87.1 D3 approved for splash and droplet protection.
- Products are made from FDA-approved PET.
- Shields are 100% recyclable.
Original Press Release:
Sinclair & Rush, Inc. Upholds Commitment to Expand Current Line of American Made PPE
Making good on their promise, US based plastics manufacturer Sinclair & Rush, Inc. releases two additional versions of their VisiGuard® face shield to the public.
Arnold, MO – June 12, 2020 - In March of 2020, as the COVID19 pandemic began rapidly spreading in the United States, employees of Sinclair & Rush, Inc. rushed to design and produce a clear plastic face shield that would not only be effective but also unique compared to others on the market.
The original VisiGuard® Face Shield was created in a way that allows it to be mass produced and shipped as economically as possible. It is made from a recyclable PET material in a heavier gauge than most other comparable shields on the market and the comfort “cups” along the top eliminate the need for any kind of additional padding, such as foam that disintegrates and cannot be sterilized. The VisiGuard® shield is resistant to chemicals like Isopropyl Alcohol, Hydrogen Peroxide, and bleach, many of the common cleaning agents found in medical environments and boasts ANSI Z87.1 D3 approval for splash, droplet, impact, and facial protection as well.
In response to customer feedback and as a direct result of the company’s dedication to offering superior products, Sinclair & Rush, Inc. is proudly releasing two additional versions of the VisiGuard® face shield: VisiGuard® 2.0 with ZipStrap® and VisiGuard® 3.0 EcoShield with ZipStrap®. The 2.0 version eliminates the latex strap in exchange for a clear, plastic strap made from the same material as the shield, keeping all the remarkable qualities of the original VisiGuard® while offering slightly more facial protection. The 3.0 duplicates the 2.0 in every way except it is smaller and thinner thus offering an alternative price point. All three versions of the shield offer ANSI Z87.1 D3 certification for splash and droplet, facial impact, and clarity. They are also FDA EUA 4132020 Model #1215 approved.
For more information on the VisiGuard® face shield, check out our website at www.visipak.com




