Thin Absorbent Skin Adhesive is suited for medical applications.
Share:
Press Release Summary:
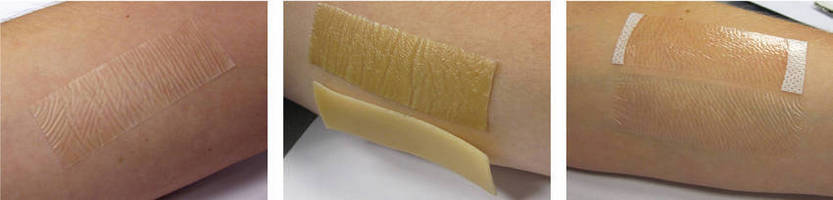
Supplied as acrylic-coated polyurethane film on polyester release liner, MED 5567A provides manufacturers of temporary adherent medical devices with alternative to acrylic adhesives and rubber-based hydrocolloid dressings. Breathability and absorbency promotes fluid handling capacity (FHC) without compromising adhesion properties and moisture vapor transmission rate characteristic of acrylic adhesives. Overall thickness is 125 µm, and transparent solution conforms to skin contours.
Original Press Release:
Avery Dennison Medical Solutions Launches Thin Absorbent Skin Adhesive with Superior Fluid Handling Capacity
MED 5567A is the Platform's First Product Offering
CHICAGO - A new medical adhesive formulation from Avery Dennison Medical Solutions, a business unit of Avery Dennison Corporation (NYSE: AVY), provides manufacturers of temporary adherent medical devices with improved performance over conventional acrylic adhesives and rubber-based hydrocolloid dressings.
The Avery Dennison® Thin Absorbent Skin Adhesive product platform leverages a patented adhesive formulation that can be used for a broad range of applications, including wound dressings, backing material for electrodes, ostomy flanges and products for direct wound contact. The platform's initial offering is MED 5567A, an acrylic-coated polyurethane film on a polyester release liner. The breathability and high absorbency of the material delivers superior fluid handling capacity (FHC) without compromising the benefits that characterize acrylic adhesives.
"The new Thin Absorbent Skin Adhesive platform will redefine market expectations for acrylic adhesives," says Barbara Van Rymenam, global market segment manager, Avery Dennison Medical Solutions. "The platform has adhesion properties and a moisture vapor transmission rate comparable to traditional acrylic adhesives, but our formulation delivers superior FHC that supports extended wear time. This also helps prevent moisture build-up to minimize skin maceration, which can make skin more susceptible to damage and can delay healing."
Wear tests comparing MED 5567A against conventional acrylic-based film dressings and rubber-based hydrocolloids reveal clean edges with minimal edge lift after three days. Its high absorption capacity can eliminate the need for a wound pad and allows continual site visualization without removing the dressing. The thin, skin-friendly adhesive has an overall thickness of 125µm and easily conforms to skin contours. Plus, it is transparent for a more discrete look compared to traditional hydrocolloids, which can be yellow in color.
To learn more, visit: http://www.medical.averydennison.com/downloads/resources/ADMS_Thin-Absorbent-Skin-Adhesive_poster.pdf
About Avery Dennison
Avery Dennison (NYSE:AVY) helps make brands more inspiring and the world more intelligent. For more than 75 years the company has been a global leader in pressure-sensitive technology and materials, retail branding and information solutions, and organization and identification products for offices and consumers. A FORTUNE 500 company with sales of $6.5 billion in 2010, Avery Dennison is based in Pasadena, California and has employees in over 60 countries. For more information, visit
www.averydennison.com
Customer Contact
Barbara Van Rymenam, T: +32 49 71 03 233
Barbara.Van.Rymenam@eu.averydennison.com
Media Relations
Bob Giuliano, T: +1 610-328-1051
Bob.Giuliano@prplace.biz
Kate Franco, T: +1 312-206-1037
Kate.Franco@averydennison.com




