Novel Revivent(TM) Myocardial Anchoring System from BioVentrix Uses Solvay's Zeniva® PEEK in Tether Component
Thermoplastic Biomaterial Delivers High Strength and Biocompatibility in New Surgical Implant for Heart Failure Patients
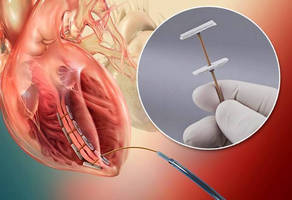
ALPHARETTA, Ga. – BioVentrix’s innovative Revivent™ Myocardial Anchoring System for the treatment of congestive heart failure features a tether component made of Zeniva® polyetheretherketone (PEEK) resin from Solvay Specialty Polymers for high strength and biocompatibility. The CE-marked heart failure therapy makes possible Less Invasive Ventricular Enhancement (LIVE™), a procedure that restores the left ventricle from damage done by a heart attack to a more optimal volume and conical shape, thereby enhancing the performance of the heart’s non-damaged myocardium and improving quality of life.
Prior to the Revivent™ System, reshaping of the left ventricle required an invasive procedure known as Surgical Ventricular Restoration (SVR), which required stopping the beating heart and supporting it with cardiopulmonary bypass while significant incisions into the heart muscle are made to excise the scarred, non-functioning (ischemic) ventricular tissue. In contrast, the LIVE™ procedure using the Revivent™ System is performed without need of a cardiopulmonary bypass or making incisions into the heart. The Revivent™ System has earned the CE (Conformité Européenne) mark which demonstrates compliance with numerous EC directives, enabling it to be sold throughout Europe. To date, more than 50 patients have been successfully treated with the Revivent™ System at leading European heart failure centers.
The unique anchoring system consists of two polyester fabric-covered anchor heads made of titanium. A flexible tether made of Zeniva® PEEK functions as a type of zip tie and is used to squeeze the anchors together and hold them in place. BioVentrix machines a flat PEEK sheet with a thickness of 0.040 in (0.1016 cm) into rectangular shapes that are 0.040 in. wide x 0.065 in. deep x and 12 in. long (0.1016 cm x 0.1651 cm x 30.48 cm). The anchor heads and tether are attached with eyelets and cam locks.
Zeniva® PEEK – part of Solvay’s line of Solviva® Biomaterials that are offered for use in implantable medical devices – has a modulus very close to that of bone combined with excellent toughness, biocompatibility, and fatigue resistance. It is certified to meet the full requirements of the ASTM F2026 standard for PEEK used in implantable surgical devices. The material was chosen over polyurethane and nylon for its strength and biocompatibility, according to Kevin Van Bladel, vice president of research and development for BioVentrix. “Zeniva® PEEK has been tested and validated for implantable devices and we are confident it will behave well in an environment where it needs to withstand the pressure of a heartbeat for the life of the patient,” explained Van Bladel.
Zeniva® PEEK has undergone fatigue testing equivalent to 15 years of simulated use and thus far two years of clinical data with patients have been gathered. BioVentrix also plans to use Zeniva® PEEK in a second-generation device, the Revivent-TC™ Myocardial Anchoring system, that uses a minimally invasive catheter approach.
Based on biocompatibility testing, Zeniva® PEEK demonstrates no evidence of cytotoxicity, sensitization, irritation, or acute systemic toxicity. It also boasts high strength and stiffness and has radiolucent properties which enable x-ray procedures without interference.
“We’ve been very pleased to work with BioVentrix as they develop improved approaches for treating heart failure,” said Dane Waund, global market manager for healthcare for Solvay Specialty Polymers. “The ongoing acceptance of Zeniva® PEEK has validated our approach to the implant market and we’re encouraged by the momentum we have generated.”
The manufacturing site for Zeniva® PEEK and other Solviva® Biomaterials in Alpharetta, Ga., is ISO 13485 registered and the relevant aspects of current Good Manufacturing Practices are also applied. Solvay’s biomaterial manufacturing processes are carefully validated and enhanced controls provide product traceability. In addition, all materials are tested in an ISO 17025 accredited lab.
In addition to Zeniva® PEEK, Solvay’s Solviva® Biomaterials line includes Proniva® self-reinforced polyphenylene (SRP), one of the world’s stiffest and strongest unreinforced thermoplastics that offers exceptional biocompatibility and hardness; Veriva® polyphenylsulfone (PPSU), which provides unsurpassed toughness combined with transparency and excellent biocompatibility; and Eviva® polysulfone (PSU), which offers good biocompatibility and practical toughness in a strong, transparent polymer. These sterilizable products are available in injection molding and extrusion grades as well as rods and plates for machined components.
About BioVentrix
BioVentrix, a privately held medical technology company headquartered in San Ramon, Calif., is focused on developing and commercializing minimally invasive as well as non-surgical therapies for treatment of heart failure. For more information, visit www.bioventrix.com.
About Solvay Specialty Polymers
Solvay Specialty Polymers is a leading global supplier of high-performance thermoplastics for permanent and prolonged exposure implants and limited exposure devices. The company has expanded its focus on the healthcare industry to meet the growing needs of its global customers by providing global technical and regulatory support. Solvay is building on its 25-year history as a key material supplier in the healthcare field, devoting considerable new resources to help customers be more efficient and cut costs. Metal-to-plastic replacement remains a key focus for manufacturers, but increased cost pressures pose a new challenge as the market continues to grow at a double-digit pace. Solvay also continues to devote considerable research and development activities to polymer technology and commercialization of new and unique material options for medical OEMs and processors.
Solvay Specialty Polymers manufactures over 1500 products across 35 brands of high-performance polymers – fluoropolymers, fluoroelastomers, fluorinated fluids, semi-aromatic polyamides, sulfone polymers, aromatic ultra polymers, high-barrier polymers and cross-linked high-performance compounds – for use in Aerospace, Alternative Energy, Automotive, Healthcare, Membranes, Oil and Gas, Packaging, Plumbing, Semiconductors, Wire and Cable, and other industries. Learn more at www.solvay.com.
As an international chemical group, SOLVAY assists industries in finding and implementing ever more responsible and value-creating solutions. The Group is firmly committed to sustainable development and focused on innovation and operational excellence. Solvay serves diversified markets, generating 90% of its turnover in activities where it is one of the top three worldwide. The group is headquartered in Brussels, employs about 29,000 people in 55 countries and generated 12.4 billion euros in net sales in 2012. Solvay SOLB.BE) is listed on NYSE Euronext in Brussels and Paris (Bloomberg: SOLB.BB - Reuters: SOLB.BR).
Press Contact:
Joseph Grande
Media Relations
413.684.2463




