New STAT-Site WB Analyzer for Use in Management of Diabetes
Press Release Summary:
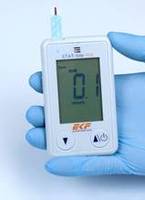
- Available with measurement range for β-ketone of 0.1 to 8.0 mmol/L and for blood glucose 10 to 600 mg/dL
- Quantitatively measure β-ketone from both fresh capillary and venous whole blood in 10 seconds
- Delivers quantitative measurements in 5 seconds for glucose in fresh capillary, venous and also neonatal whole blood
Original Press Release:
EKF Launches FDA CLIA-waived β-ketone and Glucose POC Analyzer to U.S. Market
New handheld STAT-Site WB ensures reliable and efficient analysis from whole blood
Cardiff, UK – 12th March 2020 – EKF Diagnostics, the global in vitro diagnostics company, announces a new addition to its Diabetes Care portfolio in the U.S.. The STAT-Site WB is a dual-use whole blood β-ketone and glucose meter for professional use in the management of diabetes. The new FDA CLIA-waived handheld analyzer reliably and efficiently delivers results within just 5-10 seconds, and can be used in point-of-care (POC) and Certificate of Waiver settings, such as physicians’ offices, clinics and other non-traditional laboratory locations.
As a dual analyte measurement system, the STAT-Site WB can quantitatively measure β-ketone (Beta-Hydroxybutyrate or BHB) from both fresh capillary and venous whole blood in 10 seconds. In addition, it delivers quantitative measurements in 5 seconds for glucose in fresh capillary, venous and also neonatal whole blood. Results are reported on its clear LCD screen and up to 400 can be stored in memory; these can be downloaded using a simple mini USB cable.
The new system is very simple and intuitive to use as it is reagent free, using test strips to which whole blood is added directly. These are available either individually foil-wrapped or in a vial with a shelf life of six months once opened. For easy handling, the test strips are wide and a strip indication light on the analyzer light guides insertion.
Further enhancing its ease-of-use, the STAT-Site WB’s strip identification system, automatically differentiates whether a blood glucose or β-ketone test strip has been inserted and will analyze accordingly via its auto start function. Just 1 µL of whole blood is required for this analysis and the system will detect and alert if insufficient blood is available. Also supporting inexperienced users, an automatic strip ejection function ensures that cross contamination is avoided.
Accurate with excellent linear range, the STAT-Site WB has a measurement range for β-ketone of 0.1 to 8.0 mmol/L and for blood glucose 10 to 600 mg/dL (0.5 to 33.3 mmol/L). Its wide hematocrit range is 10 to 70% for β-ketone and 20 to 70% for blood glucose. Further ensuring its accuracy, the system has auto QC identification and is calibrated using the Liquicolor® Beta-Hydroxybutyrate method. This is used by almost 1,300 hospitals in the USA to identify and monitor patients with diabetic ketoacidosis, amongst many other clinical applications.
“The introduction of STAT-Site WB further enhances our diabetes care product range. EKF Diabetes Care strives to improve patient access to diabetes care diagnostics through the provision of affordable, easy-to-use technology, reducing the cost of long-term healthcare of the growing diabetic and pre-diabetic population,” said Gavin Jones, Head of Product Management, EKF Diagnostics. “Having ready and efficient access to lab quality β-ketone and glucose results at the POC provides both practitioner and patient with the essential information they need to make clinical or lifestyle decisions in seconds.”
Due to its FDA CLIA-waived status, the new STAT-Site WB is initially only available for sale to the U.S. market. The product is currently undergoing the CE mark process and following this, this whole blood β-ketone and glucose meter will be available for professional use in the management of diabetes globally.
For more information on EKF Diagnostics, see www.ekfdiagnostics.com
About EKF Diagnostics www.ekfdiagnostics.com or www.ekfusa.com
EKF Diagnostics Holdings plc, which includes the EKF Diagnostics, Stanbio Laboratory and DiaSpect brands, specializes in the development, production and worldwide distribution of point-of-care analyzers for use in the detection and management of diabetes and anemia, and also in sports and maternal medicine. EKF products are sold in more than 100 countries around the globe, through a network of specialist distributors.
Point-of-care diagnostics: EKF Diagnostics designs and manufactures world-class diagnostic devices, as well as distributing rapid test kits for infectious diseases and pregnancy. The EKF analyzer range is used widely in GP surgeries, pharmacies, blood banks, sports clinics, hospitals and laboratories for glucose, lactate, hemoglobin, hematocrit and HbA1c measurement.
Central laboratory: EKF, through its wholly owned subsidiary, Stanbio Laboratory (Boerne, Texas, USA), manufactures a comprehensive range of clinical chemistry reagents, as well as associated analyzers. In addition, EKF Life Sciences (Elkhart, Indiana, USA) manufactures enzymes used in reagent development and also provides contract fermentation facilities.
Product Enquiries:
EKF Diagnostics Holdings plc
Martyn Lewis
t: +44 (0)29 20 710570
e: MartynLewis@ekfdiagnostics.com
w: www.ekfdiagnostics.com
Media Enquiries:
Alto Marketing Limited
Sarah Perceval
t: +44 (0)1489 557672
e: sarahp@alto-marketing.com
Twitter: @AltoMarketing
w: www.alto-marketing.com




