Bioreactor aids stem cell derived hollow organ generation.
Press Release Summary:
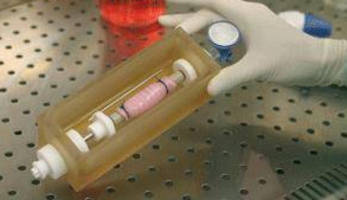
Used for tissue engineering and regenerative medicine research applications using hollow organs, InBreath Whole Organ Bioreactor is precision engineered for cell seeding and culturing on both intraluminal and extraluminal surfaces of tubular matrix. Polymer-based culture chamber houses scaffold and culture medium for entire duration of procedure. Cell/scaffold construct is rotated at adjustable 0-5 rpm on holder via brushless DC motor, which is separate from culture compartment.
Original Press Release:
New Bioreactor for Stem Cell Derived Hollow Organ Generation
Harvard Apparatus in conjunction with Dr. Paolo Macchiarini, [Macciarini, P. et al. Clinical transplantation of a tissue-engineered airway, Lancet, 372, 2023-2030, (2008)] has developed the InBreath Whole Organ Bioreactor*.
The InBreath is one of the world's first commercially available bioreactors for tubular organ regeneration. Designed for tissue engineering and regenerative medicine research applications using trachea, esophagus, intestines, blood vessels or virtually any hollow organ, the InBreath is precision engineered for cell seeding and culturing on both intraluminal and extraluminal surfaces of a tubular matrix.
A polymer-based culture chamber houses the scaffold and culture medium for the entire duration of the organ generation procedure.
An organ scaffold is mounted to the corresponding scaffold holder of appropriate diameter. Each holder features a reduced diameter central portion which functions to expose the intraluminal surface of the matrix for cell seeding and culturing.
Secondary elements or "paddles" moving with the scaffold holder produce continuous mixing of the culture medium to increase oxygenation and mass transport. The cell/scaffold construct is rotated on the holder by a brushless DC motor (0-5 rpm adjustable) which is completely separated from the culture compartment.
A co-axial conduit links the inner chamber to the external environment through an interface at the chamber wall. The detachable connection between the motor unit and the culture chamber allows the latter to be removed for maintenance while the motor unit remains stationed in the incubator.
An external control unit regulates and monitors rotation. Autoclavability, ease of handling under sterile conditions, reliability and precision ensures full compatibility of the device with GLP rules.
*For Research Use Only; not for use on humans unless proper investigational device regulations have been followed.
To receive a copy of the NEW Regenerative Medicine Catalog, go to www.harvardapparatus.com or contact
Harvard Apparatus is a global developer, manufacturer and distributor of innovative and specialized products to enhance bioresearch.




