World's Smallest Pacemaker Licenced for Canadian Patients
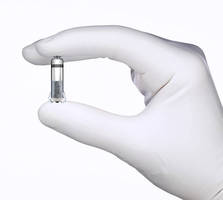
The Medtronic Micra™ Transcatheter Pacing System (TPS) is the First Leadless Pacemaker Licenced in Canada
BRAMPTON, ON, Oct. 22, 2016 - Medtronic Canada, a subsidiary of Medtronic plc (NYSE:MDT), today announced it has received a Health Canada licence for the world's smallest pacemaker, the Medtronic Micra™ Transcatheter Pacing System (TPS). The Micra TPS is the first Health Canada licenced product with miniaturized pacing technology. It is cosmetically invisible and small enough to be delivered through a catheter and implanted directly into the heart - providing a safe alternative to conventional pacemakers without the need for cardiac wires (leads).
Comparable in size to a large vitamin capsule, the Micra TPS is attached to the heart with small tines and delivers electrical impulses that pace the heart through an electrode at the end of the device. Unlike traditional pacemakers, the Micra TPS does not require leads or a surgical "pocket" under the skin, and there are no visible signs of the device. The Micra TPS responds to patients' activity levels by automatically adjusting therapy.
Micra TPS is the first and only leadless pacing system to be licenced for both 1.5 and 3 Tesla (T) full-body magnetic resonance imaging (MRI) scans, providing patients with continued access to these advanced imaging diagnostic procedures.
The Micra design incorporates a retrieval feature to enable repositioning if needed; however, the device is designed to be left in the body. For patients who need more than one device, the miniaturized Micra TPS was designed with a feature that enables it to be permanently turned off so it can remain in the body and a new device can be implanted without risk of electrical interaction.
In November 2015, data from the Medtronic Micra TPS Global Clinical Trial, which included two Canadian centres, were published in the New England Journal of Medicine and presented during a late-breaking Special Report at the American Heart Association Scientific Sessions. These data showed the Micra TPS was successfully implanted in 99.2 percent of patients, there were no (0) dislodgements, and the system met its safety and effectiveness endpoints with wide margins at six month follow-up.
Data presented in August 2016 at the European Society of Cardiology (ESC) Congress showed that the risk for major complications with the Micra TPS remained consistently low, with 96 percent of patients experiencing no major complications through 12 months follow-up (95 percent confidence interval, 94.2 percent-97.2 percent, P<0.0001). The Micra TPS reduced the risk of major complications by nearly half (48 percent; hazard ratio = 0.52, P=0.001) compared to conventional systems and the risk was lower across all patient sub-groups, whether measured by age, sex or comorbidity (all hazard ratios < 1.0).
The overall reduction in major complications with the Micra TPS was associated with a 47 percent decrease (p=0.017) in the risk of hospitalization and 82 percent (p<0.001) reduction in risk of system revisions (meaning extraction, repositioning or replacement) compared to conventional pacing systems.
"Dating back to the development of the first external battery operated pacemaker more than 60 years ago, Medtronic has a long history of collaborating with clinicians to better understand the needs of patients, and then innovating new products to meet those needs," said Michael Blackwell, director of the CardioVascular Group at Medtronic Canada. "We are thrilled to be the first to introduce a transcatheter pacemaker to patients in Canada, and we're looking forward to working with physicians and educating implanters to extend the positive results of our global clinical trial experience to even more patients."
The Micra TPS was awarded CE Mark in April 2015 and FDA approval in April 2016. It is intended for use in patients who need a single-chamber pacemaker. The device was designed to allow patients to be followed by their physicians and send data remotely via the Medtronic CareLink™ Network; remote monitoring of Micra devices is expected to be available in the near future.
In collaboration with leading clinicians, researchers and scientists worldwide, Medtronic offers one of the broadest ranges of innovative medical technology for the interventional and surgical treatment of cardiovascular disease and cardiac arrhythmias. The company strives to offer products and services that deliver clinical and economic value to healthcare consumers and providers around the world.
Multimedia Release
An animation and graphics are available by request.
About Medtronic
Medtronic Canada (www.medtronic.ca), headquartered in Brampton, Ontario is a subsidiary of Medtronic plc, which is among the world's largest medical technology, services and solutions companies - alleviating pain, restoring health and extending life for millions of people around the world. Medtronic is proud to employ over 1,600 people in Canada, serving physicians, hospitals and patients across the country. The company is focused on collaborating with stakeholders around the world to take healthcare Further, Together.
SOURCE Medtronic Canada
CONTACT:
Melicent Lavers,
Public Relations,
+1-905-460-3653;
Ryan Weispfenning,
Investor Relations,
+1-763-505-4626



