PVDF Single-Use Biocontainers feature multi-layer design.
Press Release Summary:
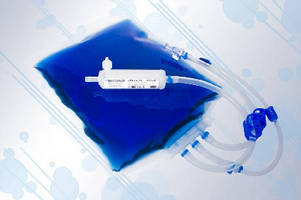
Made of multi-layer coextruded film, One-Touch® FluoroFlex® Biocontainers have PVDF product contact layer for inherent purity and are optimized to meet demanding pharmaceutical requirements. EVOH gas barrier helps mitigate risk of degradation of process solutions. Extruded, manufactured into biocontainers, and packaged in ISO Class 7 cleanrooms, FluoroFlex film is free of additives, offers thermal stability and chemical resistance, and is certified animal component free.
Original Press Release:
Industry's First Single-Use Multi-Layer PVDF Biocontainer
As the most recent addition to the One-Touch® single-use system's portfolio, Meissner introduces its new polyvinylidene fluoride (PVDF) based FluoroFlex® biocontainers. Meissner's FluoroFlex® biocontainers are made of a new generation, multi-layer coextruded film that has been optimized for the pharmaceutical industry's most demanding requirements. FluoroFlex® biocontainer applications stretch beyond that of traditional polyolefin-based biocontainers to open new opportunities for singleuse
systems.
FluoroFlex® biocontainers employ a PVDF product contact layer for its inherent purity. FluoroFlex® film is free of additives, and, therefore it offers an extremely low leachables and extractables profile. It also offers thermal stability, chemical resistance and robustness that exceed the limits of traditional PE-based biocontainers.
The FluoroFlex® biocontainer's exceptional EVOH gas barrier and its inherent purity help mitigate the risk of degradation of process solutions and API's stored and processed in the biocontainers.
Additionally, elastomeric thermoplastic (TPU) strength layers in the patent-pending FluoroFlex® film structure render it strong, yet highly flexible. FluoroFlex® film is certified animal component free (ACF). The film is extruded, manufactured into biocontainers and packaged in ISO Class 7 cleanrooms.
Meissner manufactures advanced microfiltration products and One-Touch® single-use systems used in the sterilization of drugs, reagents, and other critical pharmaceutical and biomanufacturing applications. Meissner provides comprehensive qualification and validation services, documentation, and application support to optimize filtration and single-use fluid management systems. Meissner's global customer base is serviced through a worldwide network of personnel. For more information, visit www.meissner.com, call 805-388-9911, or write: Meissner Filtration Products, Inc., 4181 Calle Tesoro, Camarillo, CA 93012.




