NIST Researchers develop test of toxin strength.
Share:
Press Release Summary:

A new NIST assay, using a glow or no glow technique, may help the US Department of Homeland Security (DHS) defend the nation against biological weapons that could be used in a terrorist attack. As part of its efforts to address this threat, DHS is working with NIST to create a standardized ricin sample with a known potency in order to check the accuracy of detection equipment and, should an attack occur, confirm the success of decontamination procedures.
Original Press Release:
NIST Researchers 'All Aglow' Over New Test of Toxin Strength
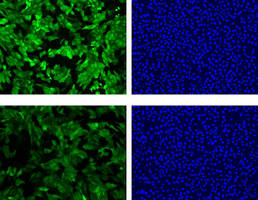
A new National Institute of Standards and Technology (NIST) assay using a "glow or no glow" technique may soon help the U.S. Department of Homeland Security (DHS) defend the nation against a spectrum of biological weapons that could be used in a terrorist attack. One very dangerous toxin on the list is ricin, a protein derived from castor beans that is lethal in doses as small as 500 micrograms-about the size of a grain of salt.
As part of its efforts to address the threat, DHS is working with NIST to create a standardized ricin sample with a known potency. Such a standard is needed both to check the accuracy of detection equipment, and, should an attack occur, to confirm the success of decontamination procedures. A major step toward this goal-the development of a rapid, reliable and precise assay of the potency of a ricin sample-has now been achieved by NIST scientists.
As detailed in an article posted online this week in Assay and Drug Development Technologies,* the new NIST assay uses a commercially available cell line genetically engineered to produce large amounts of green fluorescent protein (GFP). Ricin shuts down ribosomes-the protein manufacturing factories of a cell. Assay cells exposed to the toxin will quickly stop synthesizing GFP. This, in turn, will result in a measurable decrease in fluorescence-a drop that correlates directly to the strength of the ricin present.
The NIST assay yields many advantages over traditional cytotoxicity measuring systems, including: a highly sensitive response to ricin (as little as 1 nanogram per milliliter) within six rather than 24 hours; detection of the toxin long before significant cell death has occurred; results that are highly reproducible; no need for the addition of any reagents; and the flexibility to measure the potency of other ribosome inhibitors, even nanoparticles, with the same precision as ricin.
Partial support for this work was provided by the DHS Science and Technology Directorate.
For more information, see http://csb2.boulder.nist.gov/cstl/biochemical/cell_systems/ricin_assay.cfm.
* M. Halter, J.L. Almeida, A. Tona, K.D. Cole, A.L. Plant and J.T. Elliott. A mechanistically relevant cytotoxicity assay based on the detection of cellular green fluorescent protein. Assay and Drug Development Technologies, Vol. 7, No. 4 (August 2009; posted online the week of June 15, 2009).




